Ever wondered what makes up the world around you? Everything from the screen you’re reading this on, the air you breathe, and even your own body, all come down to one thing: atoms.
Atoms are the fundamental building blocks of everything in the universe, and understanding their structure is key to understanding the world around us.
In this article, we’ll explore the fascinating world of atomic structure, breaking it down in a simple and easy-to-understand way.
The concept of atomic structure takes us back to the very foundations of matter and its basic building units. It’s like understanding the blueprint of a building – once you understand the atomic structure, you’ll have a clearer picture of how matter works and interacts.
So, let’s dive in and unravel the mystery of atomic structure!
What is an Atom?
An atom is the smallest particle of an element that retains the properties of that element. It’s like a tiny dot in the vast universe of matter, yet it carries within it the unique characteristics of the element it represents.
Atoms are incredibly tiny – so small that millions of them could fit across the width of a single human hair. Yet, despite their minuscule size, they are composed of even smaller particles: protons, neutrons, and electrons. It is the arrangement and interaction of these subatomic particles that define the atomic structure of a particular atom.
Understanding the Basic Atomic Model
The atomic model is a theoretical representation of the structure of an atom. It helps us visualize the arrangement of protons, neutrons, and electrons within an atom.
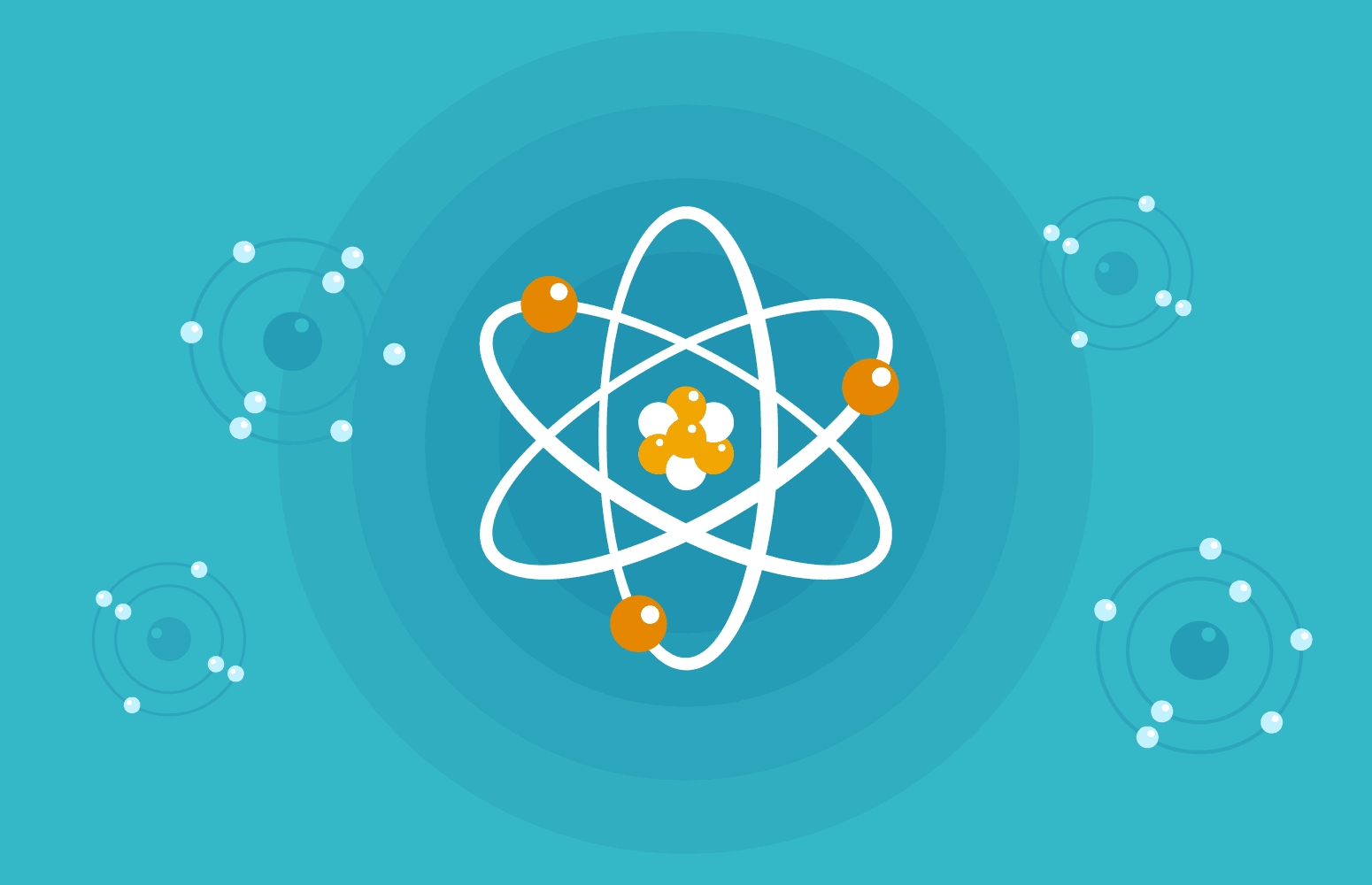
The most basic atomic model depicts the atom as having a central nucleus, made up of protons and neutrons, with electrons orbiting around it in what are known as energy levels or shells.
Imagine the atom as a tiny solar system. The nucleus is like the sun at the center, and the electrons are the planets revolving around it. The energy levels can be thought of as the orbits in which the planets move.
The atomic model helps us understand the atom’s structure and the role that each subatomic particle plays in forming this structure.
The Diagram of an Atom: A Visual Guide
Now that we have a basic understanding of what an atom looks like, let’s get a bit more visual. A diagram of an atom can be a helpful tool in visualizing the atomic structure.
In most atomic diagrams, the central nucleus is represented by a small circle or sphere, typically filled with dots or circles to represent protons and neutrons. Surrounding this are larger concentric circles or ellipses, which represent the energy levels where electrons reside.
Each energy level can hold a specific number of electrons. The first energy level closest to the nucleus can hold up to 2 electrons, the second can hold up to 8, and so on.
Understanding this diagram is essential for grasping the concept of atomic structure as it provides a visual representation of the atom’s basic components and their arrangement.
Breaking Down the Atomic Structure: Protons, Neutrons, and Electrons

Atomic structure, as mentioned earlier, is composed of three main components: protons, neutrons, and electrons. Each of these subatomic particles plays a unique role in defining the characteristics of an atom and, subsequently, the element it represents.
Protons are positively charged particles found in the nucleus of an atom. The number of protons in an atom determines the atomic number of an element, which is a fundamental characteristic of each element and distinguishes one element from another.
Neutrons, like protons, reside in the atomic nucleus, but they carry no electrical charge – they are neutral. The number of neutrons in an atom, along with the number of protons, determines the atomic mass of an element.
Electrons are negatively charged particles that orbit the nucleus in energy levels. The number and arrangement of electrons in an atom determine its chemical properties, including how it bonds with other atoms to form molecules.
The Atomic Nucleus: The Heart of the Atom
The atomic nucleus, often referred to as the heart of the atom, is the central part of the atom where protons and neutrons reside. Despite being incredibly small – accounting for just a fraction of the volume of an atom – the nucleus contains virtually all of an atom’s mass. This is because protons and neutrons are significantly heavier than electrons.
The atomic nucleus plays a critical role in holding the atom together. The positive charge of the protons attracts the negatively charged electrons, keeping them in orbit around the nucleus. This delicate balance of forces is what gives the atom its stability and allows it to exist.
The Role of Protons in the Atomic Structure
Protons, as mentioned before, reside within the atomic nucleus and carry a positive electrical charge. But what role do they play in atomic structure? Well, the number of protons in an atom fundamentally defines what that atom is.
Each element in the periodic table is defined by its atomic number, which is the number of protons in its atoms. For instance, hydrogen has one proton, so its atomic number is 1. Oxygen has 8 protons, so its atomic number is 8. This proton count is what fundamentally differentiates one type of atom – and hence one type of element – from another.
The Role of Neutrons in the Atomic Structure
Next in line are neutrons, the neutral particles residing within the atomic nucleus. Neutrons are crucial for the stability of the nucleus. They help to hold the protons together within the nucleus by offsetting the repulsive force that two positively charged protons would otherwise exert on each other.
The number of neutrons in an atom, coupled with the number of protons, determines the atom’s atomic mass. The atomic mass is essentially the total weight of an atom, and it’s measured in atomic mass units (amu), where one amu is approximately equal to the weight of a proton or a neutron.
The Role of Electrons in the Atomic Structure
Last, but certainly not least, are electrons. Electrons are tiny, negatively charged particles that orbit the nucleus in energy levels. While they contribute almost nothing to the atom’s mass (as they are nearly 2000 times lighter than protons and neutrons), electrons play a critical role in determining an atom’s chemical properties.
The arrangement of electrons in an atom’s energy levels determines how that atom can interact with other atoms. This includes how it forms chemical bonds to create molecules, which are the building blocks of everything we see around us.
The Evolution of Atomic Models Over Time

Understanding the atomic structure hasn’t always been as clear-cut as it is today. The atomic model has evolved significantly over time, with each iteration bringing us closer to the understanding we have now.
It all started with John Dalton’s solid sphere model in the 19th century, which suggested atoms were indivisible and indestructible spheres. Then came J.J. Thomson’s “plum pudding” model, which proposed that atoms were spheres of positive charge with negatively charged electrons embedded within.
Ernest Rutherford’s nuclear model followed, introducing the concept of a central atomic nucleus. However, it was Niels Bohr’s model that really refined our understanding of atomic structure, introducing the concept of energy levels for electron arrangement. This was later expanded upon by the quantum mechanical model, which is the currently accepted model and describes electrons as existing in a cloud-like probability zone around the nucleus.
How Atomic Structure Determines Chemical Properties
You might be wondering, “How does the atomic structure relate to an element’s chemical properties?” Well, the answer lies in the arrangement of electrons in an atom’s energy levels. These arrangements determine how an atom interacts with other atoms and how it forms chemical bonds.
For instance, atoms with a full outer energy level are stable and less likely to interact with other atoms. On the other hand, atoms with incomplete outer energy levels tend to readily form bonds with other atoms to achieve stability. It’s these interactions and bonds that give rise to the vast array of chemical compounds we see in the world around us.
Practical Applications of Understanding Atomic Structure
So why is understanding atomic structure important? For starters, it forms the basis of all chemical reactions and interactions. Whether it’s understanding how a particular medicine interacts with your body, why a metal rusts, or how plants perform photosynthesis, it all comes down to the atomic structure.
In the world of materials science, understanding atomic structure can help design new materials with specific properties. In medicine, it can lead to the development of new drugs. In environmental science, it can help us understand and mitigate pollution. The applications are truly endless!
Resources for Learning More About Atomic Structure
If you’re intrigued by the world of atoms and want to dive deeper, there are plenty of resources available. Online platforms like Khan Academy and Coursera offer comprehensive courses on atomic structure and chemistry.
For a more hands-on approach, consider chemistry sets or atomic model kits that allow you to physically build and visualize different atomic structures.
Books like “The Disappearing Spoon” by Sam Kean provide a fascinating and accessible glimpse into the world of atoms and the periodic table. And of course, never underestimate the power of a good old-fashioned Google search or YouTube tutorial.
Conclusion: The Significance of Atomic Structure in Our World
In conclusion, understanding atomic structure is like having a key to the universe. It opens up a world of understanding about why things are the way they are. From the air we breathe to the devices we can’t live without, it’s all because of the magical world of atoms and their structures.
So, next time you look at the world around you, remember: it’s all just a fascinating arrangement of atoms. And you are now one step closer to decoding their secret language. Happy exploring!