So, you want to dive into the world of acids and bases? Let’s start on a journey together that will take us through the everyday life of acids and bases. You might be thinking, “Wait, I didn’t sign up for a chemistry class.” Well, don’t worry, this isn’t your typical chemistry lecture. This adventure is full of everyday examples, fun facts, and even some experiments that you can try at home.
Acids and bases are everywhere in our lives, from the food we eat to the products we use. They play a critical role in many processes, including digestion, cleaning, and even the functioning of our bodies. And you know what? Understanding them is actually a lot simpler than you might think. So, put on your lab coat, grab your safety goggles, and let’s get started.
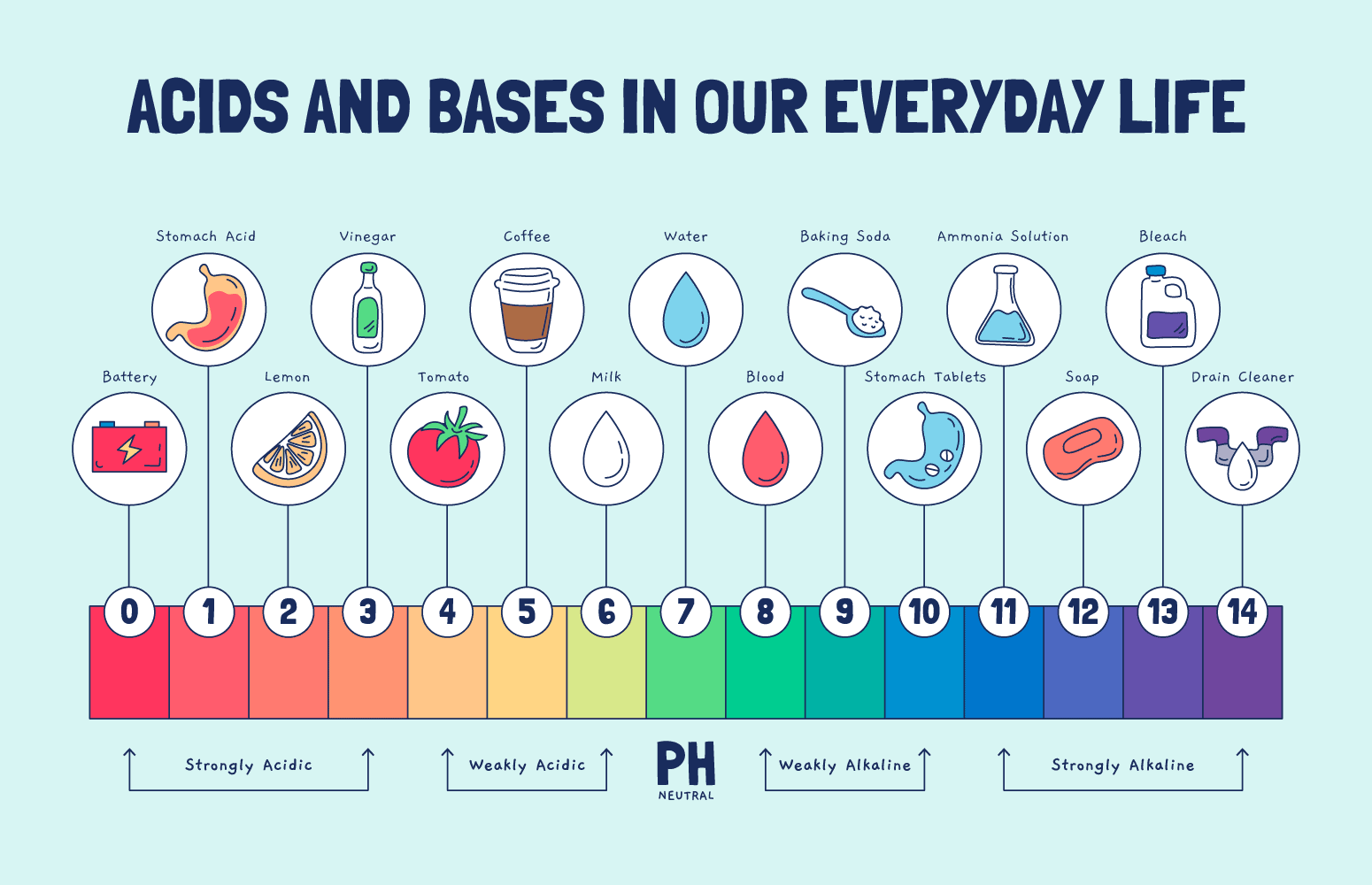
Understanding the pH Scale
Before we dive into the deep end, let’s get to know our companion for this journey – the pH scale. Imagine the pH scale as a sliding scale from 0 to 14 that tells us how acidic or basic (also called alkaline) a substance is. A pH of 7 is neutral, like pure water. Anything below 7 struts into the acid side of town, while anything above 7 dances over to the base side.
Now, don’t be fooled by the numbers. The pH scale isn’t a regular scale. It’s a logarithmic one. That means each step is ten times more than the last. So, a pH of 3 is ten times more acidic than a pH of 4 and a hundred times more acidic than a pH of 5. Mind-blowing, isn’t it?
10 Most Common Acids in Our Daily Life
Now that we’ve got the basics down, let’s explore the interesting world of acids in daily life.
1. Citric Acid: This is found in a variety of fruits, most notably citrus fruits like oranges, lemons, and limes. It’s also used as a natural preservative in a variety of food products and is a common ingredient in sodas and other beverages due to its tangy flavor. For example, citric acid is a crucial ingredient in products like Sprite and 7 Up.
2. Acetic Acid: Acetic acid is the primary component of vinegar, contributing to its strong, sour taste and pungent smell. This is often used in pickling, marinating, and salad dressing recipes.
3. Lactic Acid: This acid is found in dairy products such as sour milk, buttermilk, yogurt, and cottage cheese. It’s also produced in our muscles during intense exercise, causing the feeling of muscle burn.
4. Ascorbic Acid: Better known as Vitamin C, ascorbic acid is found in a variety of fruits and vegetables, including oranges, strawberries, kiwi, bell peppers, and broccoli. It is necessary for growth, development, and repair of body tissues.
5. Sulfuric Acid: Although it is not directly consumed, sulfuric acid is used in the production of phosphoric acid, a common ingredient in sodas like Coca-Cola and Pepsi. Furthermore, it’s used in the manufacture of fertilizers, so it indirectly contributes to our food production.
6. Hydrochloric Acid: This is a strong acid produced by our stomach to aid in digestion. It helps break down food and kill bacteria.
7. Tannic Acid: Found in tea, coffee, and in some types of fruit, tannic acid contributes to the bitter taste and is believed to have various health benefits including antioxidant properties.
8. Salicylic Acid: This acid is commonly used in skincare products to treat conditions such as acne, psoriasis, dandruff, and warts.
9. Carbonic Acid: Present in carbonated drinks, such as soda and sparkling water, carbonic acid is what gives these drinks their fizz. It’s formed when carbon dioxide is dissolved in water.
10. Malic Acid: Found in apples and many other fruits, including apples, pears, cherries, and plums. It has a tart flavor that is often used to enhance the taste of food and drinks. Malic acid is also used as an ingredient in many skincare products because it can help exfoliate skin and reduce inflammation.
8 Most Common Bases in Our Daily Life
Time to flip the coin and take a look at bases in daily life. Bases, like acids, are all around us, but they’re often more subtle. You won’t taste a base in your food the way you taste an acid, but you’ll find them in many household products.
1. Sodium Bicarbonate (Baking Soda): Sodium bicarbonate, commonly known as baking soda, is a key ingredient in many baking recipes. It helps in leavening bread, cakes, and pastries by producing carbon dioxide gas during the cooking process.
2. Calcium Hydroxide (Lime): Lime is often used for adjusting the pH level in soil and water purification systems. It is also used in the production of cement and mortar.
3. Magnesium Hydroxide (Milk of Magnesia): This is a common over-the-counter medication used to neutralize stomach acid and treat symptoms such as heartburn, acid reflux, and constipation.
4. Sodium Carbonate (Washing Soda): Sodium carbonate is a common ingredient in laundry detergents, acting as a water softener by binding hard water minerals. It’s also used for swimming pool maintenance to control the acidity of the water.
5. Sodium Hydroxide (Lye): Sodium hydroxide, also known as lye, is an essential component in soap making. It’s also used in drain and oven cleaners due to its ability to break down proteins and fats.
6. Calcium Carbonate (Chalk): Chalk, made from calcium carbonate, is used in classrooms for writing on blackboards and in sports like gymnastics and rock climbing to improve grip. It’s also an active ingredient in antacids to help neutralize stomach acid.
7. Potassium Hydroxide (Potash): This is used in a variety of industries, from soap making to fertilizer production and food processing. It’s also used in biodiesel production.
8. Ammonia: Ammonia is a base used in many household and industrial cleaners. It’s also used in the production of fertilizers and in the refrigeration industry as a cooling agent.
Remember, while these bases play important roles in our daily lives, some of them can be harmful if not used correctly. Always handle such products according to their safety guidelines to protect your health.
Safety Precautions When Handling Acids and Bases
While acids and bases are part of everyday life, they’re not always fun and games. Handling strong acids or bases requires safety precautions. Always wear gloves and eye protection when handling these substances.
If you spill an acid or base, don’t try to clean it up with your bare hands. Use a neutralizing agent, like baking soda for an acid or vinegar for a base.
Remember, never mix acids and bases unless you know what you’re doing. Some combinations can produce dangerous gases or heat. As a general rule, always add acid to water, not the other way around. This prevents the acid from splashing and causing burns.
Impact of Acids and Bases on Health
While acids and bases are invaluable parts of our lives, an imbalance can have health implications. For instance, a diet high in acidic foods can cause acid reflux and even erode tooth enamel. Similarly, exposure to strong bases, like bleach or ammonia, can cause skin and eye irritation.
On the flip side, the right balance of acids and bases is essential for our health. Our bodies constantly work to maintain a slightly alkaline pH of around 7.4. This balance allows enzymes to function properly, helps transport oxygen throughout the body, and keeps our cells healthy.
Acid-Base Reactions We Encounter Daily
We encounter acid-base reactions every day, often without realizing it. Have you ever noticed how baking soda and vinegar fizz when mixed together? That’s an acid-base reaction. The vinegar (acid) reacts with the baking soda (base) to produce carbon dioxide gas (the fizz) and water.
Or consider antacids. When you take an antacid for heartburn, the base in the antacid neutralizes the excess stomach acid, relieving your symptoms. That’s an acid-base reaction too. Even your body’s process of maintaining a stable pH, called homeostasis, involves constant acid-base reactions.
Intriguing Facts about Acids and Bases

Before we wrap up our journey, here are some intriguing facts about acids and bases.
Did you know that the strongest acid in the world is fluoroantimonic acid? It’s so strong it can dissolve glass!
The strongest base, ortho-diethynylbenzene dianion, can strip protons from a glass surface, effectively dissolving it.
Ever heard of acid rain? It’s rain with a lower pH due to atmospheric pollution. Acid rain can harm plants and aquatic life.
On the other end of the spectrum, alkaline water, with a higher pH, is marketed for its supposed health benefits, although the evidence is still inconclusive.
Conclusion: Acids and Bases in the Everyday World
As we wrap up our journey, it’s clear that acids and bases are integral parts of our everyday world. They’re in the food we eat, the products we use, and even in our own bodies. Understanding them not only opens up a world of scientific knowledge but also helps us appreciate the complex and beautiful chemistry of our daily lives.
So next time you bite into a tangy apple, clean your kitchen, or even brush your teeth, remember the amazing world of acids and bases that make it all possible. And who knows? Maybe you’ll be inspired to conduct your own experiments or explore more about these fascinating substances.